Types of Study
Introduction to Types of Medical Research
Evidence-based medicine may be defined as the systematic, quantitative and preferentially experimental approach to obtaining medical information. This information is obtained through medical research. Medical research encompasses a wide range of study techniques that can be used to understand diseases, uncover their causative factors and validate the treatments we have available for them. Each type of study technique comes with advantages as well as their own particular disadvantages. This article will introduce the different types of study commonly used within medical research and discuss their particular traits. The diagram below provides an overview of how the different types of study methodology relate to one another.
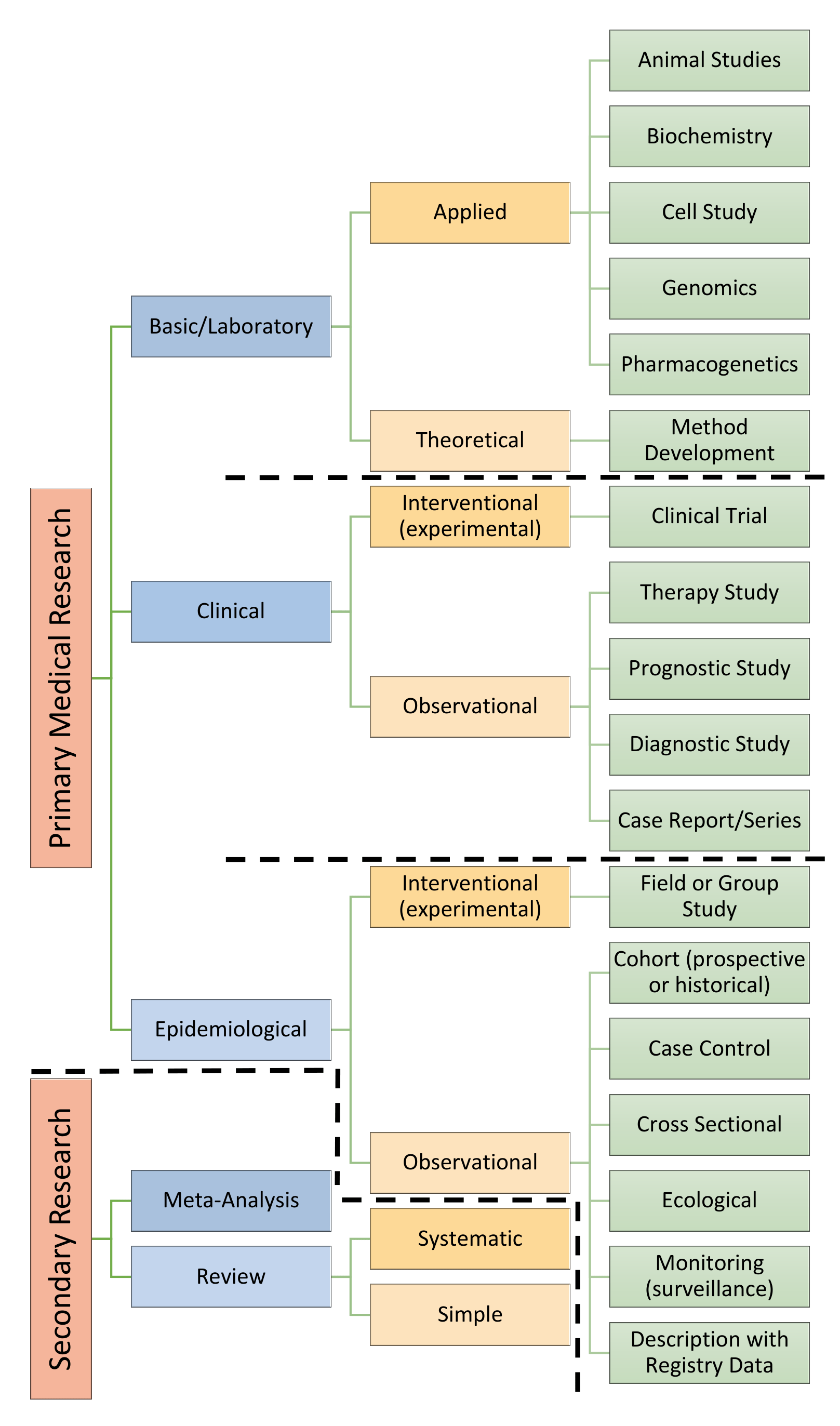
Primary vs. Secondary Research
Medical research can be classified as either primary or secondary research. Primary research involves performing studies and collecting raw data. Secondary research involves evaluating or synthesising data collected during primary research.
Observational vs. Experimental Research
An observational study is a study in which the investigator does not seek to control any of the variables nor the assignment of intervention to subjects. These decisions are usually made by the patient and their doctor. Examples include cohort, case-control, case-series and cross-sectional studies.
An experimental study involves direct manipulation or assignment of participants to different interventions or environments. Clinical experimental studies are known as clinical trials.
Prospective vs. Retrospective
In prospective studies, individuals are followed over a period of time and data is collected when their characteristics or circumstances change. Studies usually relate the outcome of interest to suspected risk factors. For these prospective studies, the outcome of interest should commonly occur to ensure statistical significance. Prospective studies allow precise estimation of the relative risk of an outcome based upon exposure.
In retrospective studies, individuals are sampled and information is collected about their past. These studies usually establish an outcome of interest and examine exposures to suspected risk or protective factors. Data is typically gathered from interviews or medical notes. The nature of retrospective studies makes them more susceptible to bias. Retrospective studies allow calculation of the odds ratio (this is an estimate of the relative risk) for uncommon outcomes. Retrospective studies are advantageous for studying rare diseases since prospective studies are unfeasible due to the large study sizes needed to reach statistical significance.
Randomised vs. Non-Randomised
Randomised studies involve the random allocation of individuals to intervention groups in order to minimise confounding variables. Allocation does not take into account any similarities or differences in the individuals. It usually involves use of a random number generator.
Non-randomised studies involve allocation of people to different interventions using methods which are not random.
Single-Blinded vs. Double Blinded vs. Triple-Blinded
Blinding is important to reduce bias and ensure a study’s internal validity. It prevents participants and researchers from affecting the outcomes of a study in a conscious or subconscious manner.
- Single-blind study – only the participants are blinded.
- Double-blind study – both participants and experimenters are blinded.
- Triple-blind study – participants, experimenters and researchers analysing the data are blinded.
The Levels of Evidence
Not all evidence is created equal with some forms of study technique thought to be superior in design. Studies which employ superior designs are felt to carry more weight when interpreting their conclusions. The result is the creation of a hierarchy based upon study technique. This has been outlined in the diagram below.
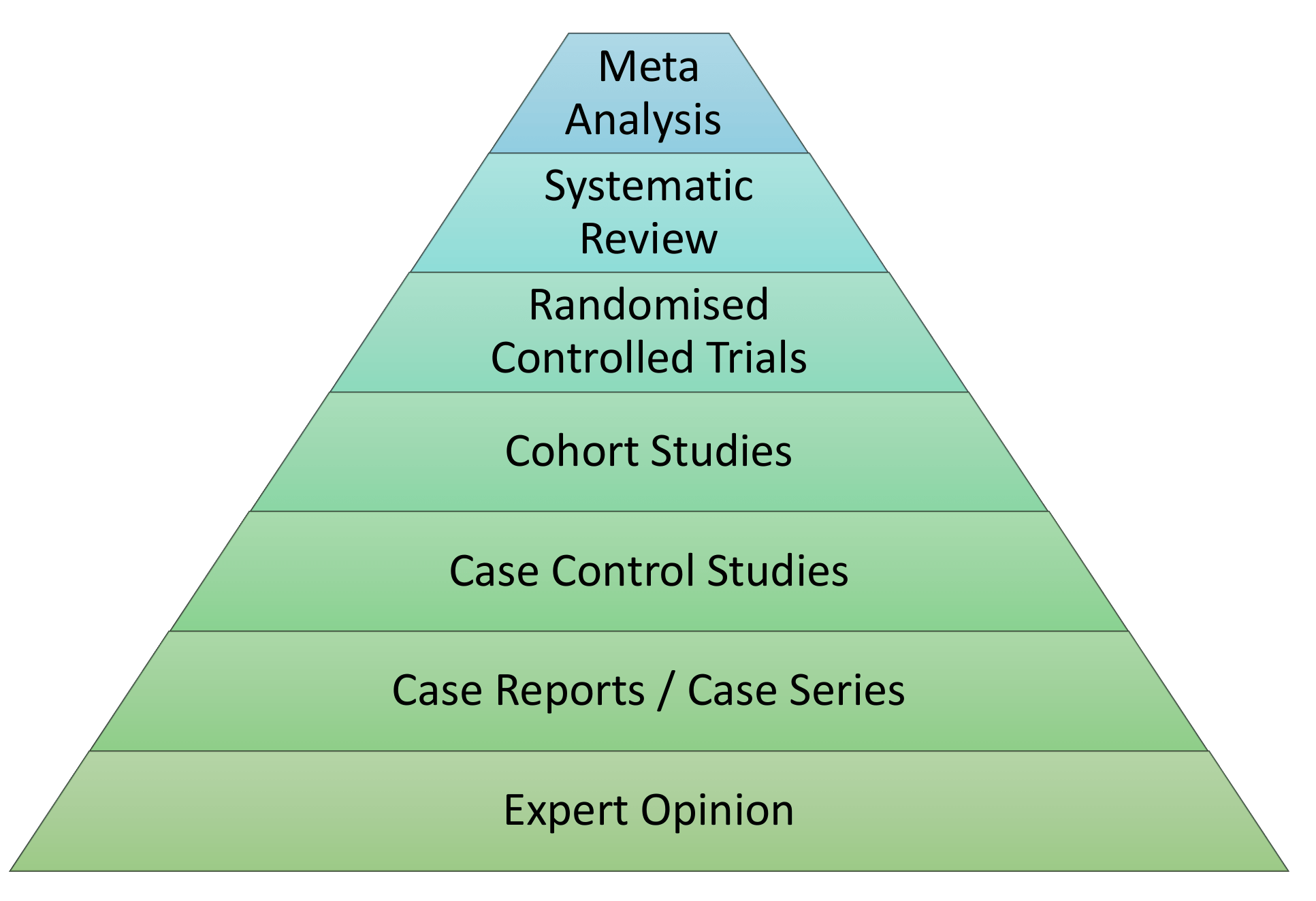
The ordering of evidence in this manner may be seen as simplistic because it does not take into account the methodological merit of individual study designs. Furthermore, the quality of systematic review evidence will depend largely upon the type of study included within the analysis and meta-analysis results can vary wildly depending upon the statistical methods employed. In the final instance, systematic reviews should be considered a lens through which evidence can be viewed.
Brief Description of Study Types
In this section we will cover the basics of the following study designs.
- Meta-Analysis
- Systematic Review
- Randomised Control Trial
- Cohort Study
- Case Control Series
- Case Report/Series
For further information on each of these study designs and how to perform them, have a look at the Equator Network.
Meta-Analysis - Secondary Research
Definition: A meta-analysis is a statistical procedure for systematically combining numerical (quantitative) data from multiple independent studies in the published literature. These data are assessed and used to derive conclusions about that body of research. It is a subset of systematic reviews (see below).
Uses: Meta-analyses can be used to provide more precise estimates than those given by any individual study included within the analysis. They may also answer questions not posed by individual studies or identify and examine the heterogeneity between the individual studies (including statistical significance where conflicting results are reported). Examples of alternative questions include providing a more complex analysis of harms/benefits or the examination of subgroups where individual study numbers were not large enough.
Brief Methodology: The Cochrane collaboration has developed a protocol which provides structure for literature search, analytic and diagnostic methods for evaluating the output of meta-analyses. These can be viewed within their handbook. Additional guidance can be found by using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses). This is an evidence-based minimum set of items (checklist) for reporting in systematic reviews and meta-analyses.
Advantages
- Provides greater statistical power and increased volume of data for more precise estimates.
- Hypothesis testing and biases within publications can be examined.
- Inconsistencies within research can be resolved.
- Provides better estimate of relationships.
Disadvantages
- It is difficult and time consuming to identify the correct studies.
- Not all studies may be appropriate for inclusion.
- An incomplete set of studies may have been analysed.
- Requires advanced statistical capabilities.
- Heterogeneity of methods used in studies may lead to erroneous inferences.
Systematic Review - Secondary Research
Definition: A systematic review is a detailed, systematic and transparent means of considering all published and unpublished material which fits within a prespecified eligibility criteria. The included material can be of varying study designs. Those materials which are judged to be methodologically sound are combined in either a quantitative or qualitative manner to answer a pre-defined research question. Meta-analyses are not required but many systematic reviews will include a meta-analysis.
Uses: Systematic reviews are used to deliver a meticulous summary of the available primary research in response to a research question.
Brief Methodology: Systematic reviews should have a clear set of objectives, predefined eligibility criteria, a reproducible methodology, a systemic search method, an assessment of the validity of the findings of included studies and a systematic presentation and synthesis of the attributes and findings from the studies used.
Brief Methodology: The Cochrane collaboration has developed a protocol which provides structure for literature search, analytic and diagnostic methods for evaluating the output of meta-analyses. These can be viewed within their handbook. Additional guidance can be found by using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses). This is an evidence-based minimum set of items (checklist) for reporting in systematic reviews and meta-analyses.
Advantages
- Addresses a specific question.
- Explicit and bias limiting methods.
- More reliable and accurate than individual studies.
- Less costly than organising a new study.
- Requires less time than a new study.
- Results can be generalised and extrapolated into the general population.
Disadvantages
- Time consuming.
- There may be difficulties combining different studies.
- May be composed of inadequate primary studies.
- May be poorly designed and executed.
- May mis-interpret results.
Randomised Controlled Trial - Primary Research, Experimental, Prospective
Definition: A randomised control trial involves one or more new treatments where participants are randomly assigned into an experimental or control group. The various groups are then followed up to see if there is any difference in the specified outcome. The results and subsequent analysis are used to evaluating the effectiveness of the intervention.
Uses: Randomised controlled trials are used to establish the effectiveness of a new intervention or treatment.
Brief Methodology: Interventions might include a medication or procedure. Control groups will either get a placebo treatment or receive the current ‘gold standard’ treatment. Randomisation seeks to evenly distribute baseline characteristics in order to reduce the effect of confounding variables. This process is usually performed using mathematical techniques.
The CONSORT (Consolidated Standards of Reporting Trials) Statement can be used as an evidence-based minimum set of recommendations (checklist) for reporting randomised trials. The Cochrane Library has formed a highly concentrated source of reports of randomised controlled trials which can be found within their CENTRAL (Cochrane Central Register of Controlled Trials) database.
Advantages
- You can make direct comparisons between treatments.
- Effective randomisation removes selection bias.
- Randomisation reduces the impact of confounding factors and makes groups comparable with both known and unknown factors.
- Results can be reliably analysed with statistical tools.
- Blinding can be applied to reduce performance bias.
- Prospective design minimises recall error and selection bias.
Disadvantages
- It is expensive and takes time.
- Participants must volunteer and so may not be representative of the whole population.
- Studies will have to be powered sufficiently to make significant outcomes.
- There is the risk of participants being lost to follow up.
- Ethical limitations. For example, informed consent is impossible to obtain, or some intervention arms would be ethically impossible.
- Results may not mimic realise and generalisability to the real world may be difficult.
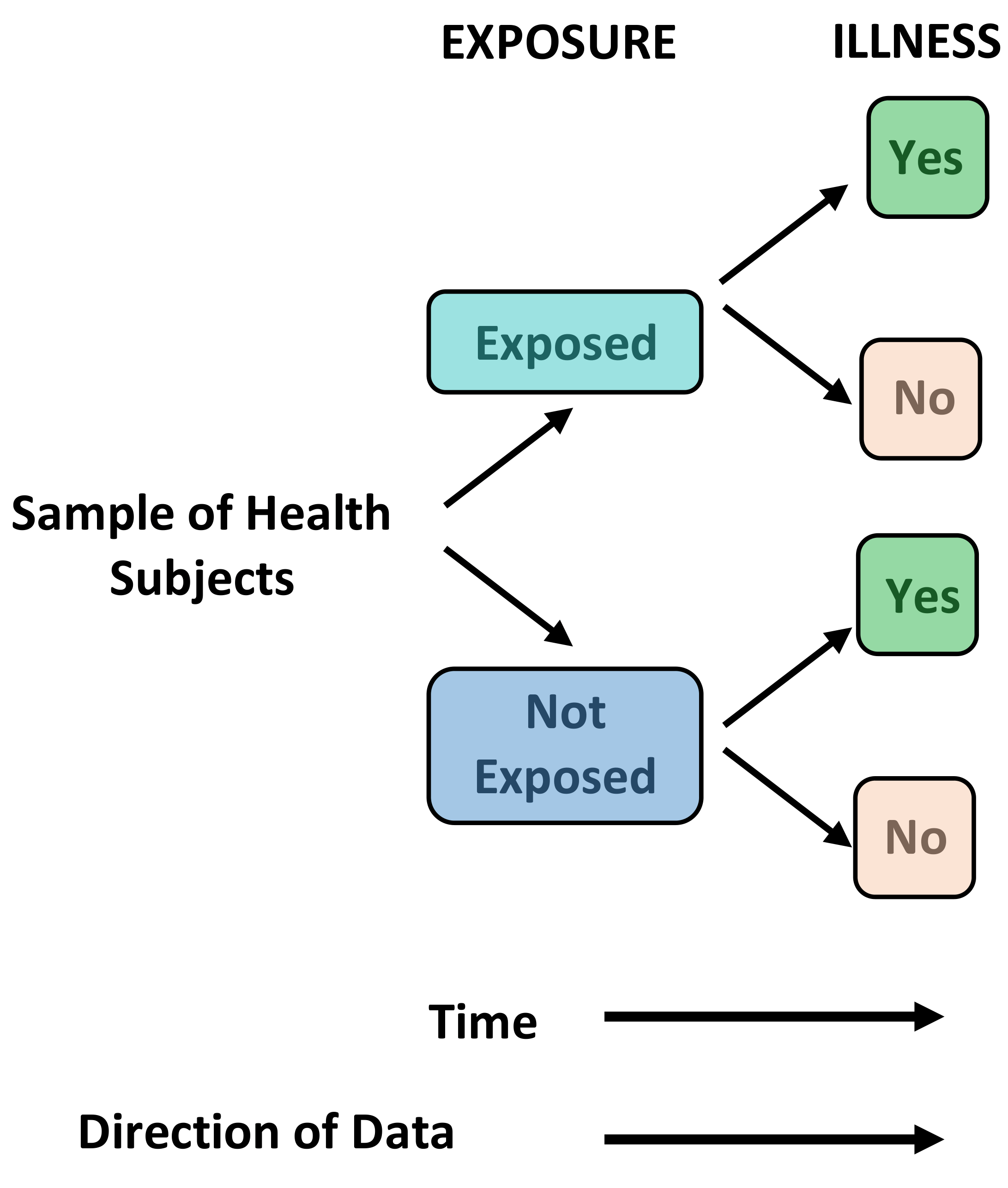
Cohort Studies - Primary Research, Observational, Predominantly Prospective
Definition: Groups of disease-free individuals are identified, and baseline measurements are taken for a variety of variables (risk factors) that might be relevant to the development of the outcome of interest. These individuals are then followed over time to determine whether they develop the outcome of interest. Cohort studies are usually prospective but can be performed retrospectively with data collected for other purposes.
Uses: Cohort studies measure incidence rates and the relative risk for developing the outcome of interest for each measured variable. They are able to distinguish between cause and effect due to the temporal relationship between risk factor exposure and outcome occurrence.
Brief Methodology: In prospective cohort studies the risk exposure information is collected at the start of the study and new cases of disease identified from that point onwards. In retrospective cohort studies the exposure status was measured in the past and disease identification has already begun. Both methods enable calculation of the relative risk.
The STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) statement (checklist) can be used to ensure observational studies are adequately described in research publications. This checklist has been designed for cohort studies, case-control studies and cross-sectional studies.
Advantages
- It is cheaper and easier to implement than a randomised controlled trial.
- It is able to distinguish between cause and effect.
- Multiple outcomes can be studied.
- It may uncover unanticipated associations with the outcome.
- The efficiency of prospective cohort studies increases as the incidence of any particular outcome increases.
Disadvantages
- Patients can be lost to follow up thereby introducing attrition bias.
- Subject selection can introduce bias due to an imbalance of patient characteristics.
- It is prone to change of methods over time.
- Confounding variables can be difficult to remove.
- It is difficult to blind researchers.
- Requires large numbers of patients.
- The outcome of interest can take a long time to occur.
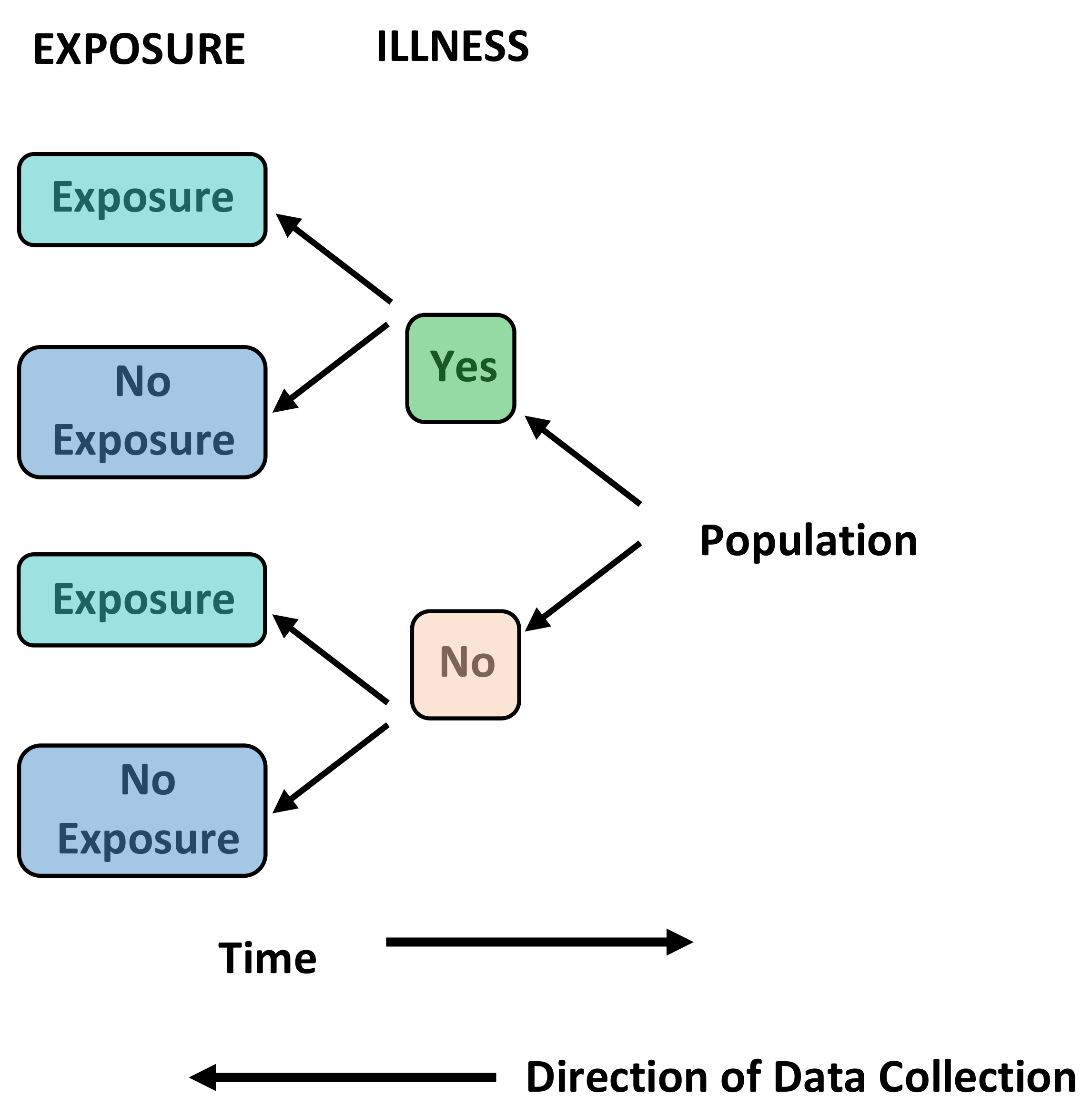
Case Control Studies - Primary Research, Observational, Retrospective
Definition: A study that compares patients who have an outcome of interest (the disease in question) with those who do not. Case control studies are almost always retrospective. The researcher looks back in time to identify which individuals were exposed to a risk factor or treatment and thus the relation it has with the presence or absence of disease.
Uses: Good for studying rare diseases and outcomes. They can also be used where there is a long latent period between an exposure and disease occurrence. They are often used to generate hypotheses that can then be studied using other means.
Brief Methodology: Individuals with the outcome of interest (the disease in question) are selected (cases). A second group of similar individuals without the outcome of interest is constructed (controls). The researcher then looks at historical factors to identify if some exposures are found more commonly in the cases than the controls. If this is the case, a link can be established between the exposure and the outcome of interest. This produces an odds ratio that can be used to approximate the relative risk for each variable studied.
The STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) statement (checklist) can be used to ensure observational studies are adequately described in research publications. This checklist has been designed for cohort studies, case-control studies and cross-sectional studies.
Advantages
- Requires less time to perform the study as the disease has already occurred.
- It allows you to simultaneously look at multiple risk factors.
- It can be used to establish initial associations for further research down the line.
- Relatively cheap.
- Quick to complete.
- Small numbers are required.
- Control groups are simple to organise.
Disadvantages
- Data is prone to recall bias.
- Control group prone to selection bias.
- Case group prone to sampling bias.
- Confounding can easily occur (exposure and outcome are both strongly associated with a third variable).
- Only one outcome can be studied (presence or absence of the disease).
Case Report/Series - Primary Research, Observational, Retrospective
Definition: An article that describes and interprets an individual case or cases. It is often written as a detailed story.
Brief Methodology: An interesting case is identified, and the patient should be described in detail. Include the following: their age, sex, ethnicity, race, employment status, social situation, medical history, diagnosis, prognosis, previous treatments, diagnostic tests, medications, current intervention and the clinical and functional assessment.
Uses: Describe unique cases that cannot be explained by known diseases or syndromes. They may show an important variation from a known disease. They may show unexpected events that yield new information. They may include patients with two or more unexpected diseases or disorders.
Advantages
- Can help identify new trends or diseases.
- They can help detect new drug side effects and potential uses.
- They are an educational way of sharing lessons learned.
- They can identify rare manifestations of a disease.
Disadvantages
- Cases may not be generalisable.
- The causes or associations may have other explanations.
- They can be seen to emphasize the bizarre or focus on misleading elements.
References
- Stats Direct
- Cochrane Handbook
- CONSORT Statement: Cosolidated Standards of Reporting Trials
- PRISMA Statement: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- STROBE Statement: Strengthening the Reporting of Observational Studies in Epidemiology
- Equator Network: Enhancing the Quality and Transparency of Health Research
- Open MD: Medical Research
- Deutsches Ärzteblatt International: Types of Study in Medical Research
- Himmelfarb: Types of Studies
- Georgia State University: Literature Reviews: Types of Clinical Study Designs
- Deutsches Ärzteblatt International: Systematic Literature Reviews and Meta-Analyses
- Emergency Medicine Journal: Randomised Controlled Trials and Their Principles